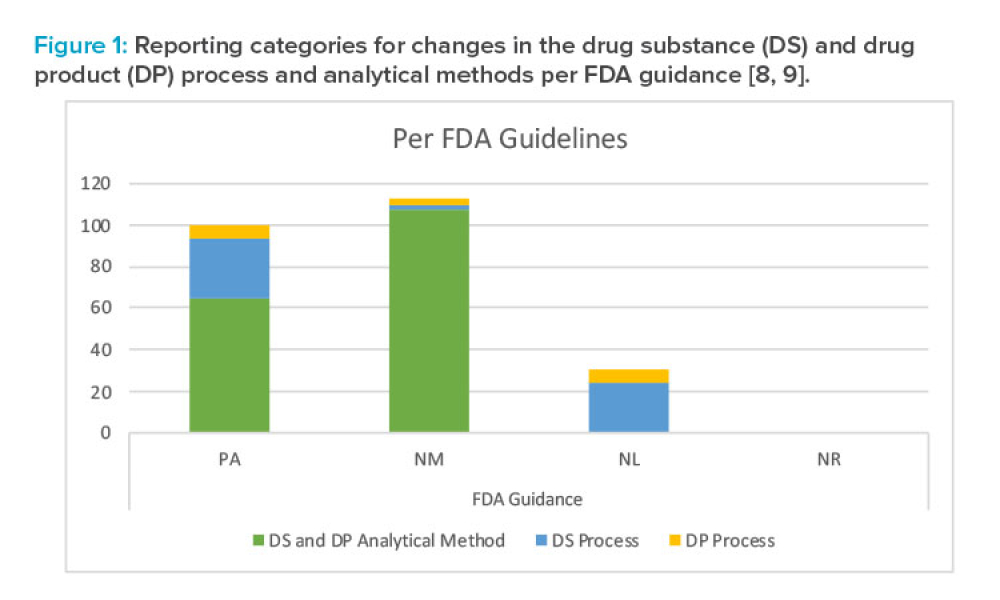
As the demand for accelerated access to medicines expands globally, the pharmaceutical industry is increasingly submitting regulatory applications in multiple countries simultaneously. As a result, Boards of Health (BoHs) are challenged with approving these applications in an accelerated timeframe and accommodating the submission of postapproval chemistry, manufacturing, and controls (CMC)...
NOTICE: Our system upgrade is now live. As we roll out new features, you may experience some intermittent issues today. We appreciate your patience as we work to resolve them. Thank you for your understanding.